Keywords:
urofollitropin, urofollitropin’s R&D Progress, Mechanism of Action for urofollitropin, drug target for urofollitropin.
Description:
This article summarized the latest R&D progress of urofollitropin, the Mechanism of Action for urofollitropin, and the drug target R&D trends for urofollitropin.
Text:
urofollitropin‘s R&D Progress
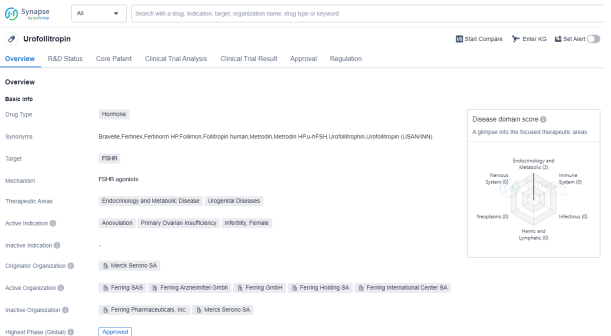
Urofollitropin is a hormone drug that targets the FSHR (follicle-stimulating hormone receptor). It is primarily used in the treatment of various conditions related to endocrinology and metabolic diseases, as well as urogenital diseases. The active indications for Urofollitropin include anovulation, primary ovarian insufficiency, infertility in females.
The drug was developed by Merck Serono SA, a well-known pharmaceutical organization. Urofollitropin has reached the highest phase of development which is approved globally. It was first approved in the United States in September 1986, making it a well-established drug in the market.
In China, Urofollitropin is currently in phase 3 of clinical trials, indicating its progress towards potential approval in the country. This suggests that the drug may soon be available to patients in China, pending successful completion of the trial and regulatory approval.
Urofollitropin is classified as an orphan drug, which means it is intended to treat rare diseases or conditions that affect a small number of patients. This regulatory designation grants drug developers benefits, including extended market exclusivity and financial support.
Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for urofollitropin: FSHR agonists
FSHR agonists are a type of drug that activate the follicle-stimulating hormone receptor (FSHR). From a biomedical perspective, FSHR agonists are used in the field of reproductive medicine to stimulate the production of follicles in the ovaries and promote ovulation in women. These drugs mimic the action of the natural hormone FSH, which plays a crucial role in regulating the growth and development of ovarian follicles.
By binding to and activating the FSHR, FSHR agonists stimulate the growth of multiple follicles, increasing the chances of successful ovulation and subsequent fertility. They are commonly used in assisted reproductive technologies such as in vitro fertilization (IVF) to enhance the recruitment and maturation of multiple eggs.
FSHR agonists can also be used in the treatment of certain hormonal disorders, such as polycystic ovary syndrome (PCOS), where there is an imbalance in the levels of reproductive hormones. By stimulating the FSHR, these agonists help regulate the hormonal environment and promote normal follicular development.
It is important to note that the use of FSHR agonists should be carefully monitored by healthcare professionals, as excessive stimulation of the ovaries can lead to ovarian hyperstimulation syndrome (OHSS), a potentially serious condition. Close monitoring and adjustment of dosage are necessary to minimize the risk of complications.
In summary, FSHR agonists are drugs that activate the follicle-stimulating hormone receptor, promoting the growth and development of ovarian follicles. They are primarily used in reproductive medicine to stimulate ovulation and increase fertility in women.
Drug Target R&D Trends for urofollitropin
FSHR, or follicle-stimulating hormone receptor, plays a crucial role in the human body’s reproductive system. Located on the surface of ovarian follicles and testicular Sertoli cells, FSHR is responsible for binding to follicle-stimulating hormone (FSH). This interaction triggers a cascade of events that are essential for follicular development in females and spermatogenesis in males. FSHR activation stimulates the growth and maturation of ovarian follicles, leading to the release of mature eggs during ovulation. In males, FSHR activation promotes the production of sperm cells. Understanding the role of FSHR is vital for developing targeted therapies and interventions to address infertility and other reproductive disorders.
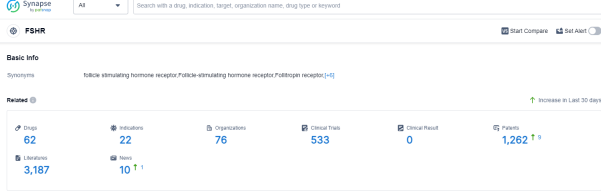
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 62 FSHR drugs worldwide, from 76 organizations, covering 22 indications, and conducting 533 clinical trials.
The analysis of the current competitive landscape of target FSHR reveals that companies like Merck KGaA, Ferring Holding SA, Merck Europe B.V., and Merck & Co., Inc. are leading in terms of R&D progress. These companies have multiple drugs in various stages of development, with Merck KGaA having the highest number of approved drugs. The most common indications for drugs targeting FSHR are infertility and infertility in females. Hormones and biosimilars are the drug types progressing most rapidly, indicating intense competition. China, the European Union, and the United States are the countries/locations developing fastest under the current target, with China showing significant progress. Overall, the target FSHR presents a competitive landscape with diverse therapeutic areas and promising future development potential.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Urofollitropin is a hormone drug developed by Merck Serono SA. It targets the FSHR and is primarily used in the treatment of endocrinology and metabolic diseases, as well as urogenital diseases. The drug has been approved globally since 1986 and is currently in phase 3 of clinical trials in China. Its active indications include anovulation, primary ovarian insufficiency, and female infertility. Urofollitropin is classified as an orphan drug, providing certain advantages to the developer.